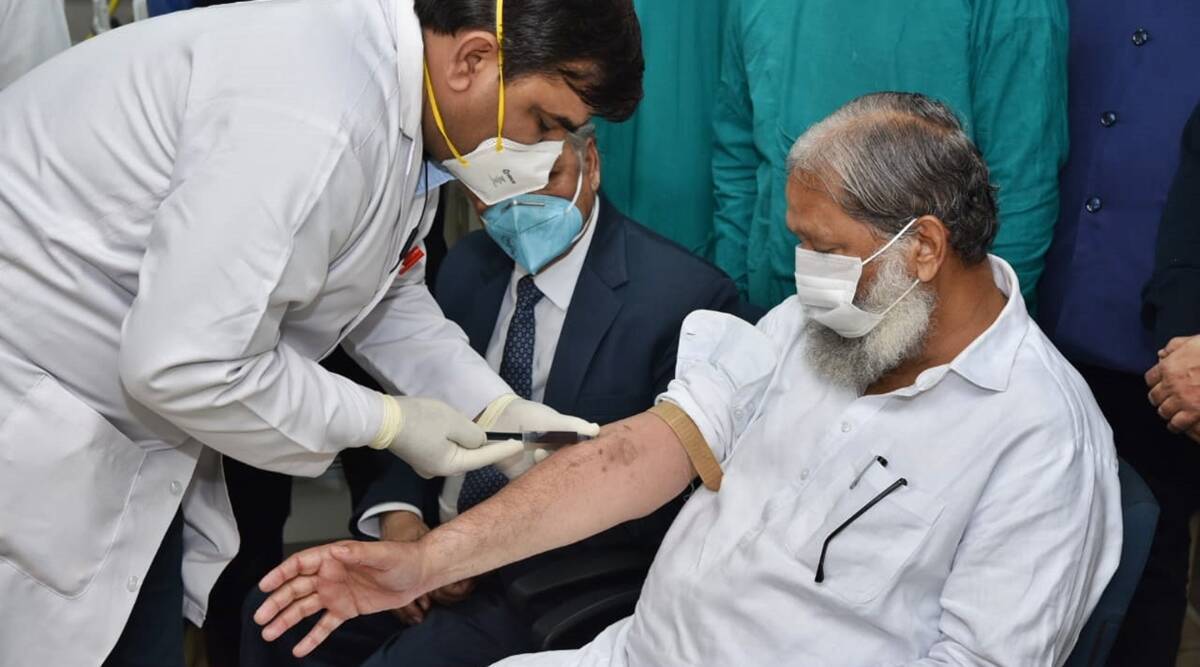
Anil Vij who heads Haryana’s Home, Health, Urban Local Bodies, Technical Education, Science & Technology Ministries had announced he has tested Corona positive. It was only on November 20, that Vij had volunteered to be the first to get a shot Covaxin, when the third phase trial started in the state. He had taken the first shot in this final round of vaccine trail that he said will be tested on 25,800 people. The vaccination shot was given in the presence of scores of TV news cameras and had been covered widely.
#WATCH Haryana Health Minister Anil Vij being administered a trial dose of #Covaxin, at a hospital in Ambala.
He had offered to be the first volunteer for the third phase trial of Covaxin, which started in the state today. pic.twitter.com/xKuXWLeFAB
— ANI (@ANI) November 20, 2020
And now Anil Vij confirmed he has tested positive for Covid-19 and is now admitted for treatment at the Civil Hospital Ambala Cantt. He asked those who met him resented to get themselves tested as well.
I have been tested Corona positive. I am admitted in Civil Hospital Ambala Cantt. All those who have come in close contact to me are advised to get themselves tested for corona.
— ANIL VIJ MINISTER HARYANA (@anilvijminister) December 5, 2020
Trial for third phase of Covaxin a coronavirus vaccine product of Bhart Biotech to start in Haryana on 20th November. I have offered myself as first volunteer to get vaccinated .
— ANIL VIJ MINISTER HARYANA (@anilvijminister) November 18, 2020
It may be recalled that last month, the family of a 40-year-old man who suffered a severely adverse reaction after participating in a clinical trial for another Covid-19 vaccine COVISHIELD, had sued the Serum Institute of India (SII) demanding Rs 5 crores as damages. The family served a legal notice to SII that is conducting the trial in association with the Indian Council of Medical Research (ICMR). The notice was served to the CEO of SII and the Director General of ICMR, and also to the Drugs Controller General of India, CEO, Astra Zeneca UK as well as Professor Andrew Pollard Chief Investigator, Oxford Vaccine Trial (The Jenner Institute Laboratories University of Oxford) as well as Vice Chancellor Sri Ramachandra Higher Education and Research.
According to the legal notice, the participant is an independent business consultant and had signed up for the trial as he is “a public-spirited person and is concerned about the healthcare system in the society, particularly its impact on the poor and the disadvantaged.” It says, “The death of millions of people all over the world due to the attack of the Covid-19 virus has affected him emotionally” and that “when he came to know that there was a call for volunteers for the 3rd phase of the human trial in Sri Ramachandra Institute of Higher Education and Research (formerly called Sri Ramachandra Medical College & Research Institute – SRMC) for testing the Covid-19 vaccine developed by the Oxford University, UK, the public spirit in him wanted to volunteer.”
The notice stated that it was frequent and repeated assertions that the vaccine was safe, that made the participant sign up for the trial and even sign a consent form on September 29, 2020. He was administered the vaccine on October 1, 2020. The participant started experiencing severe headaches and nausea, on October 11, 2020 following which there was also a change in his behaviour. It says that “he was not aware of his surroundings, he showed irritation towards light and sound, and was resisting any effort to make him get up from bed.”
Meanwhile, as scores of good wishes for Vij’s speedy recovery poured in from his followers, some have asked if Covaxin, being developed indigenously by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR), had been proven ineffective. As reported widely, the vaccine manufacturer had said it had successfully completed interim analysis of Phase 1 and 2 trials before initiating Phase 3 trials. Vij had then announced that he would be administered a trial dose at the Civil hospital, Ambala Cantt under the supervision of doctors from PGI Rohtak and Health Department. “I have volunteered to take the trial dose,” he had said.
Sad you tested positive, get well soon!
Just asking, same trial which was to be generated to “Bihar free vaccine” during election JUMLA..
Modi vaccine? or Water vaccine?
Kismat par pad gayi Modi Jumla ki laathi? first volunteer in your state for the anti-Covid vaccine?— sunitajadhav (@sunmor2901) December 5, 2020
सूचना तो चिंतित करने वाली है परन्तु आश्वस्त हूँ आप अपनी प्रचंड ऊर्जा से किसी भी रोग विकार को सफलतापूर्वक मात देंगे।
ईश्वर से आपके शीघ्र स्वास्थ्य लाभ की कामना करता हूँ।
अपने स्वास्थ्य का ध्यान रखिये व शीघ्र स्वास्थ्यलाभ प्राप्त कीजिये।@cmohry— Sanjay Tandon ?? (@SanjayTandonBJP) December 5, 2020
This is 2nd time …in short span of 4 months?. Pl get your test repeated,as antibodies hv life of atleast 3-4 months.
— Dr KBMohan:UCC? (@bkukDr) December 5, 2020
This is the Haryana Health Dept’s Covid-19 tally:
This is the official overall tally in India
Related:
COVISHIELD vaccine trial participant sues Serum Institute
Covid-19 vaccine hits road bump, as India speeds towards 100,000 daily cases